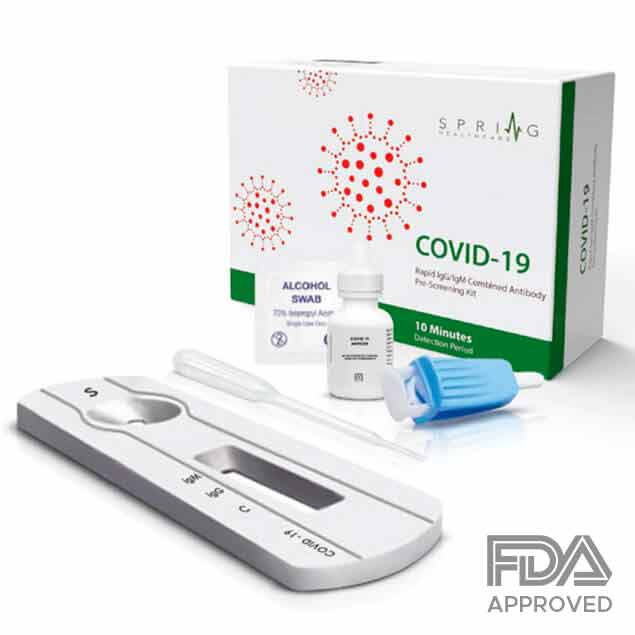
COVID-19 Rapid Test
Made in Switzerland, the FDA approved CoronaVirus Rapid Test can check a patient for the Coronavirus antibodies in 10 minutes. By checking for two Corona antibodies through a patient’s self-taken blood sample, the test can identify current and previous infections in patients, as well as levels of immunity. While most accurate Corona tests require laboratory equipment which can delay results for several hours to days, the Corona Virus Rapid Test needs no additional materials to be completed. While there have been other tests in China as well as other countries that have surfaced with similar rapid tests methods, none have proven to be accurate enough for screening public patients and providing proper diagnosis.
Rapit test 1-10 units $169
Rapit test 11-20 units $165
Rapit test 21-50 units $143
Rapit test 51-or more units $116
$169 (1-10 units) $165 (11-20 units) $143 (21-50 units) $116 (51 or more units)The FDA approved CoronaVirus Rapid Test from Switzerland has shown to have over 98% accuracy results, with several controls and sample tests having been performed prior to its recent release. With the all new, FDA and SGS approved CoronaVirus Rapid Test, screening patients will no longer involve long waits, large costs, and inaccurate results. Our test allows professionals to quickly screen any patients with symptoms while allowing the opportunity for patients to test themselves from the comfort of their home without having to come in contact with other individuals and risk further spread. At US Ophthalmic, we are putting all our resources to fight this global pandemic; we know that this test will help us overcome this challenge by keeping patients properly diagnosed and safe.
