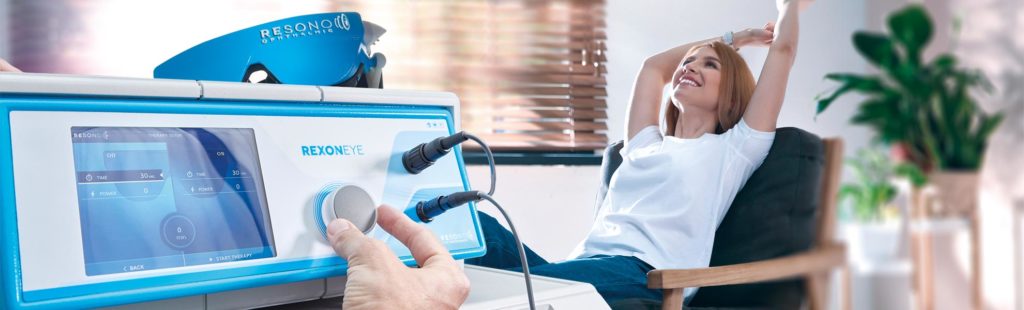
Rexon-Eye® is the only device based on QMR® technology (Quantum Molecular Resonance) for the treatment of pathologies in the ophthalmology field that may be caused by cell malfunction of some organs.
This is the case with the lacrimal and Meibomian glands in dry eye disease.
By stimulating the metabolism and natural regeneration of these cells, Rexon-Eye can restore their correct physiological behavior.

How Rexon-Eye is made
The Rexon-Eye device consists of:

A specific generator, capable of producing alternating electric current with a specific patented frequency spectrum, between 4 MHz and 64 MHz, which constitutes the QMR signal.
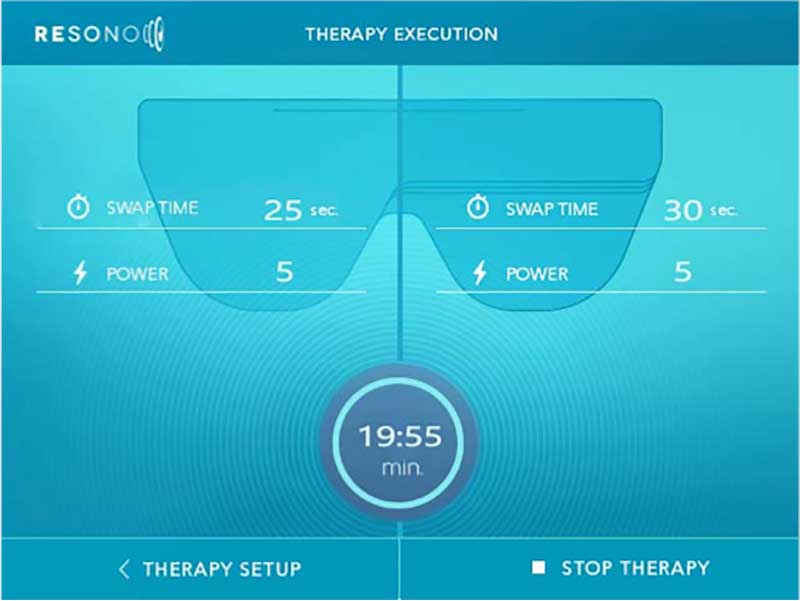
A simple graphical user interface, that allows the specialist to quickly and easily set the treatment parameters and start the therapy.

A neutral plate, positioned on the seat in contact with the body that allows for a closed electrical circuit.
A suitably designed mask, to be worn over the lids with eyes closed, which contains the two application electrodes, one for each eye.
How Rexon-Eye works
The electric field generated by Rexon-Eye, applied to the ocular surface by means of the mask electrode, stimulates and accelerates the natural regeneration processes that take place in tissues.
The treatment protocol envisages:
- 20-minute sessions, in which the eyes are stimulated individually, alternating every 30 seconds
- 4 sessions, once a week, over a total period of 4 weeks
The power is initially set to a value of 4 and may then be adjusted separately for each eye.
Rexon-Eye therapy is non-invasive, does not cause any pain, does not require analgesic products or external cooling systems.
For effective therapy, follow the advice on the Treatment page.


Results and benefits
The Rexon-Eye device is used successfully in patients with dry eye, of a hyposecretive, evaporative or mixed type.
QMR treatment promotes the regeneration of the Lacrimal Functional Unit (LFU), thus improving the activity of the glandular tissues involved.
The induction of natural tears in a greater quantity and of better quality leads to a physiological rebalancing of the tear film, with significant clinical benefits and a considerable improvement in the patient’s state of well-being.
For further information go to the Clinical Studies page.
As with any other type of therapeutic agent, the patient’s response to Rexon-Eye therapy is characterized by individual variability:
some patients experience improvement in their condition soon after the first session, for example, with increased tear production
others must wait longer for the treatment to start the natural regeneration process.

In 2016, Rexon-Eye obtained the EC certification mark as a ‘Medical device for the treatment of ocular surface disorders’ and, in 2018, after further clinical studies, it entered the therapeutic devices market.
The device is present in the National List of Medical Devices of the Italian Ministry of Health as a Class IIa device in the CND ‘Instrumentation for therapeutic and surgical treatments in ophthalmology.’

Rexon-Eye is currently patented in Europe, China, US, Russia, South Africa, Australia, Korea, and is pending in other countries.